Theories of Covalent Bond Formation
Theories of Covalent Bond Formation: Overview
This topic covers concepts, such as Valence Bond Theory, Comparison of Sigma and Pi Bonds, Valence Shell Electron Pair Repulsion Theory, Geometry of Molecules Containing Two Bond Pairs and One Lone Pair, Descending Order of Repulsive Interaction of Electron Pairs, Geometry of Molecules Containing No Lone Pair of Electrons, Formation of H2 Molecule According to Valence Bond Theory, Directional properties of Bonds, Overlapping of Atomic Orbitals, Types of Overlapping in Sigma and Pi Bonds, Formation of Sigma and Pi bonds in Ethene & Formation of Sigma and Pi bonds in Ethyne etc.
Important Questions on Theories of Covalent Bond Formation
Which of the following plot represents potential energy of a pair of nuclei as a function of their separation:
The pair of species having identical shapes for molecules of both species is –
Number of sigma bonds in is :
The number of bonds, bonds and lone pair of electrons in pyridine, respectively.
Given below are two statements :
Statement I : and both possess V-shaped structure
Statement II : The bond angle of is less than that of .
In the light of the above statements, choose the most appropriate answer from the options given below:
Statement 1: both have bent structures.
Statement 2: have less angle than .
The correct order of the bond angles in and is
A single bond is always a '' bond where as multiple bonds contain bolh and bonds. A double bond contains one and and one bond whereas a triple bond contain one and bonds. On basis of this statement calculate and bonds in .
Calculate the number of and bonds in -propylpent--ene.
Among the following, which pair of orbitals are involved in sigma bond formation (assume x-axis as internuclear axis)
If axis is taken as internuclear axis then pair of orbitals that form pi bond is
Consider the following figures and select the incorrect order of bond angle?
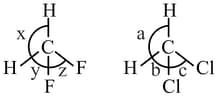
Which among the following have five sigma bond pairs and zero lone pairs according to VSEPR theory.
Lateral overlap of orbitals leads to the formation of _____.
What is the geometry of methane according to VSEPR theory.
Which one of the following statement is not correct for sigma-and pi-bonds formed between two carbon atoms?
When a chemical bond between two atoms is formed, the potential energy of the system
The number of sigma and pi bonds in ethyne molecule are respectively,
The repulsive interaction is the highest between
The repulsive interaction of electron pairs follows the order
